Abstract
Background: Adult T-cell leukemia/lymphoma (ATLL) is an aggressive mature peripheral T-cell neoplasm caused by HTLV-1 infection. Despite aggressive therapy, ATLL carries a very poor prognosis. Current prognostic models (i.e., the International Prognostic Index [IPI] and the Prognostic Index for T-cell lymphoma [PIT]) have shown to not completely predict survival in ATLL (Phillips, Cancer, 2010). Moreover, these models have not been validated in Latin American ATLL patients. Therefore, we aim to validate current prognostic systems (IPI, PIT) and to develop a novel model to predict early mortality in patients with ATLL.
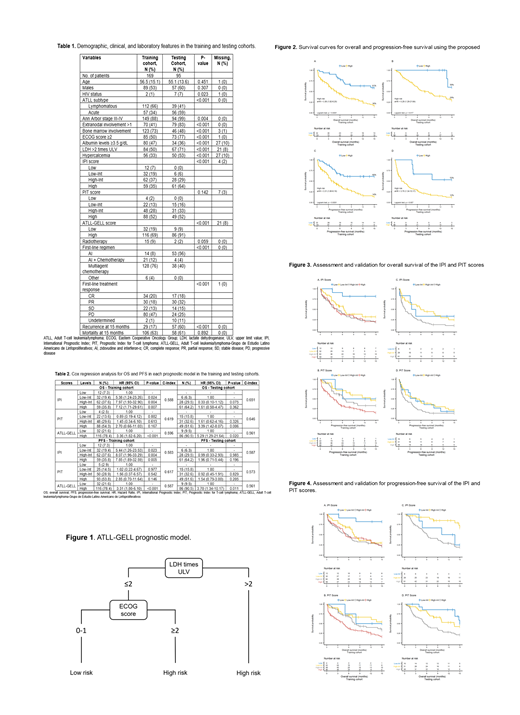
Methods: We conducted a retrospective cohort study by analyzing 12 demographic, clinical, and laboratory variables of 169 Latin American ATLL patients with acute and lymphomatous subtypes recruited by the "Grupo de Estudio Latinoamericano de Linfoproliferativos" (GELL). All patients received cancer-directed treatment. Conditional inference trees were used to construct a new prognostic model, the ATLL-GELL, by including serum lactate dehydrogenase (LDH) and the Eastern Cooperative Oncology Group (ECOG) performance status. Our primary outcome was overall survival (OS) at 15 months, while our secondary outcome was progression-free survival (PFS). The model identified a low-risk (LDH ≤2 times upper limit value [ULV] + ECOG score 0-1), and a high-risk (LDH >2 times ULV + ECOG score ≥2; or LDH >2 times ULV) group (Figure 1). The model was validated in an independent cohort of 95 North American ATLL patients with acute and lymphomatous subtypes from Miami, FL. We used the Kaplan-Meier method and the log-rank test to estimate the survival probabilities for the score. Cox regression was used to evaluate the risk of mortality and disease progression for each score. We used the C-index to determine the discriminatory power of each score.
Results: Table 1 summarizes the demographic and clinical characteristics. The mean age was 57 (± 15.1) years with a male predominance (53%). Lymphomatous ATLL was the most common subtype (66%) in the training cohort, while acute ATLL (59%) in the testing cohort. Most patients had an Ann Arbor stage III-IV (88%) and received multiagent chemotherapy (76%) as first-line treatment (Table 1). With a median follow-up of 21 months, the 15-month OS and PFS rates of the training cohort were 29% and 27%, respectively. Patients with a high-risk ATLL-GELL score had a worse OS (16% vs. 61%, p<0.0001) and PFS (14% vs. 60%, p<0.0001) (Figure 2). Table 2 shows that the ATLL-GELL model had comparable prognostic discrimination than the IPI and PIT scores, according to the C-index. Similarly, the Cox regression analysis identified a better risk stratification of the new model for OS and PFS in the training and testing cohorts. In contrast, poor risk stratification for mortality and disease progression was found with the IPI and PIT scores in the training cohort. Table 2 shows that the risk for mortality and disease progression was higher in the high-intermediate (HR 7.91 and 8.07, respectively) than the high-risk (HR 7.12, and 7.85, respectively) group according to the IPI score. Moreover, the PIT score could not properly stratify patients' OS and PFS risk (Table 2). Figures 3 and 4 illustrate narrow and crossing survival curves between risk subgroups in both scores for OS and PFS estimates, respectively, suggesting poor visual discrimination of the IPI and PIT scores.
Conclusion: ATLL carries a dismal prognosis, therefore we hypothesized that current prognostic systems might not yield an appropriate risk stratification since almost universally aggressive ATLL patients are at high risk for rapid disease progression and death. After considering different clinical-epidemiological and laboratory variables, our simplified prognostic model was able to predict early mortality and disease progression in patients with aggressive ATLL. Poor performance status, thus unfit for aggressive therapy, along with a high disease burden seems to reliably predict mortality in these patients. The ATLL-GELL model also showed better risk stratification and a more clear distinction of low- versus high-risk groups for aggressive ATLL than traditional models. We are currently validating our findings in a prospective cohort of Latin American ATLL patients and to improve clinical decision-making in those deemed at high risk for early mortality.
Castillo: Abbvie: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy; Roche: Consultancy; TG Therapeutics: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal